Overview
Research in our group is focused on measuring intermolecular and intersurface forces in complex fluid systems with an emphasis on polymers, polyelectrolytes, biomembranes, and biomimetic materials in order to develop new materials with useful properties. These materials are being studied since they can be prepared from renewable resources, can be biocompatible and biodegradable, and often possess excellent physical properties.
Microfabricated Fixed-Target Supports for Protein Crystallography
Over the last decade, brilliant, coherent femtosecond X-ray free-electron lasers (10^12 photons/pulse) have revolutionized structural biology by enabling ultrafast, room-temperature protein structure and dynamics studies. Using a diffract-before-destroy approach, even weakly-diffracting micro or nanocrystals of hard-to-crystallize proteins can be studied, but challenges exist in efficiently delivering thousands of crystals to the X-ray beam while maintaining crystal integrity in a vacuum environment. Microfabricated fixed-target supports are an exciting alternative to widely used liquid jet-based technologies as they offer distinct advantages like significantly lower sample consumption, control over sample distribution, and the ability to incorporate stimuli like ligands, caged reactants, or electric fields “on-chip” for dynamic time-resolved experiments.
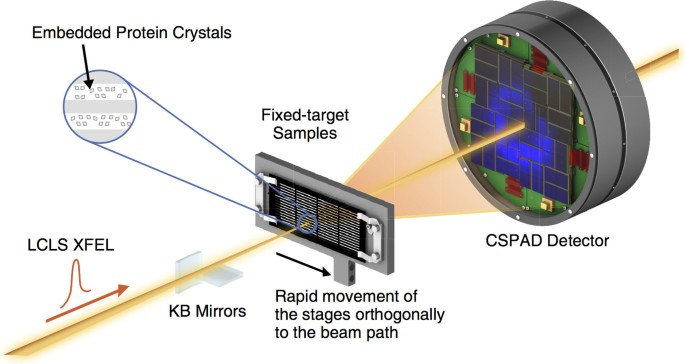
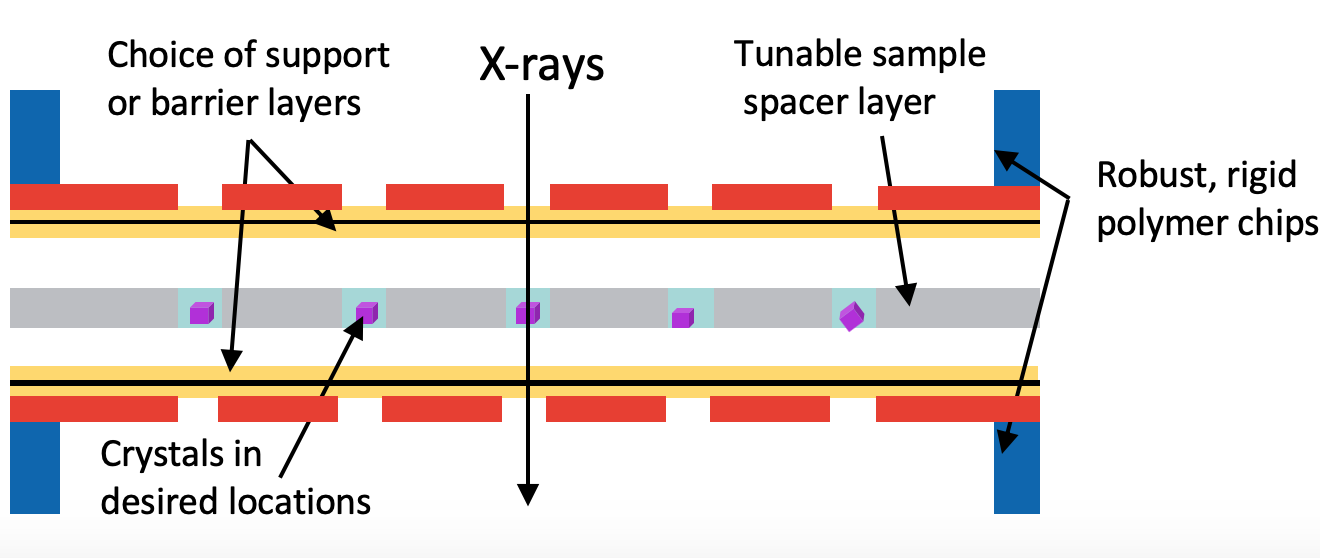
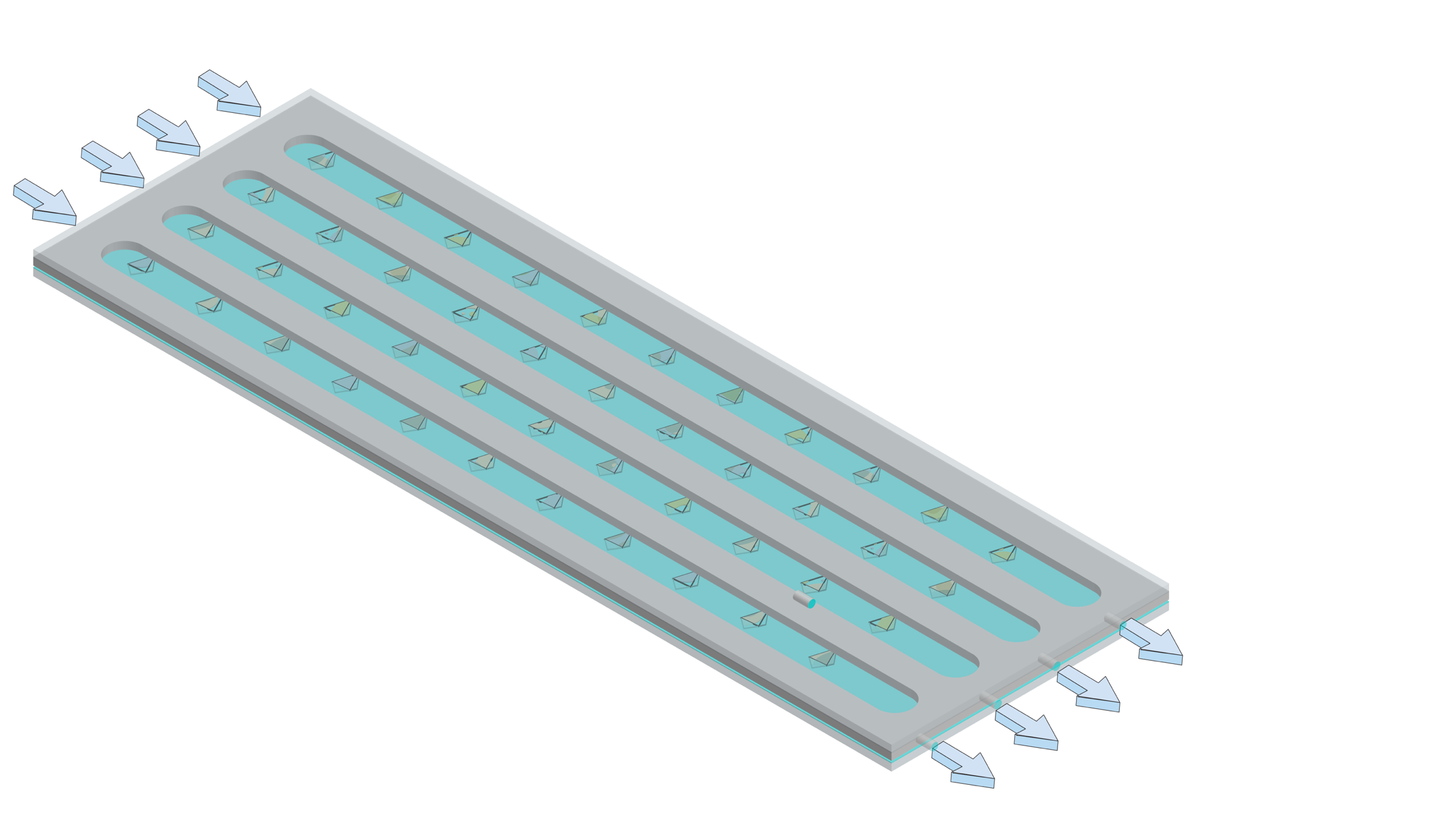
This work focuses on the development of robust, hot-embossed cyclic olefin copolymer (COC) fixed-target supports with ultra-thin, tunable water barrier films made from COC and graphene. These supports are inexpensive, maintain protein crystal hydration over extended periods of time, while contributing minimally to the X-ray scatter background compared to existing silicon or polymer-based supports. The enclosing COC thin films can be functionalized and patterned using UV-initiated photo-grafting of acrylic acid monomers, which can be further derivatized to create different charge or affinity binding moieties that act as “universal” protein crystal nucleation sites. This enables precise control of protein crystal sample distribution on fixed targets that can drastically improve sample hit-rates and reduce data collection time to a few minutes. Specific protein binding-driven crystal nucleation provides an alternative strategy for protein crystallization, particularly for proteins that are difficult to express and crystallize or have slow crystallization kinetics.
Polymers and Polymer Bonded Explosives
- Nuclear warheads are made of a uranium core surrounded by a polymer-bonded explosive, which is made of explosive nanocrystals embedded in a matrix of polymers. The matrix helps the explosive detonate in just the right way so it compresses the uranium and makes it fissionable.
- Polymers are sensitive to temperature and the environment and can degrade over time, slowly moving away from the explosive crystallite and forming voids in the material. These voids change the interface between the polymers and crystallites, creating hotspots and increasing explosive sensitivity. Keeping track of these voids is crucial for making sure warheads are both safe and usable.
- We want to study these interfacial phenomena to predict the explosive material’s behavior. If we know what’s going on, we can optimize it and make polymer-bonded explosives that are safer and longer-lasting
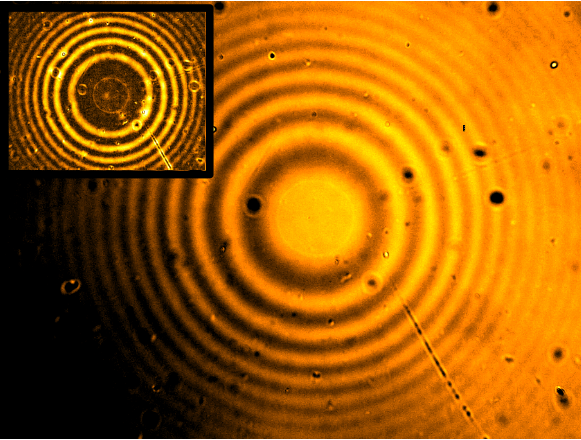
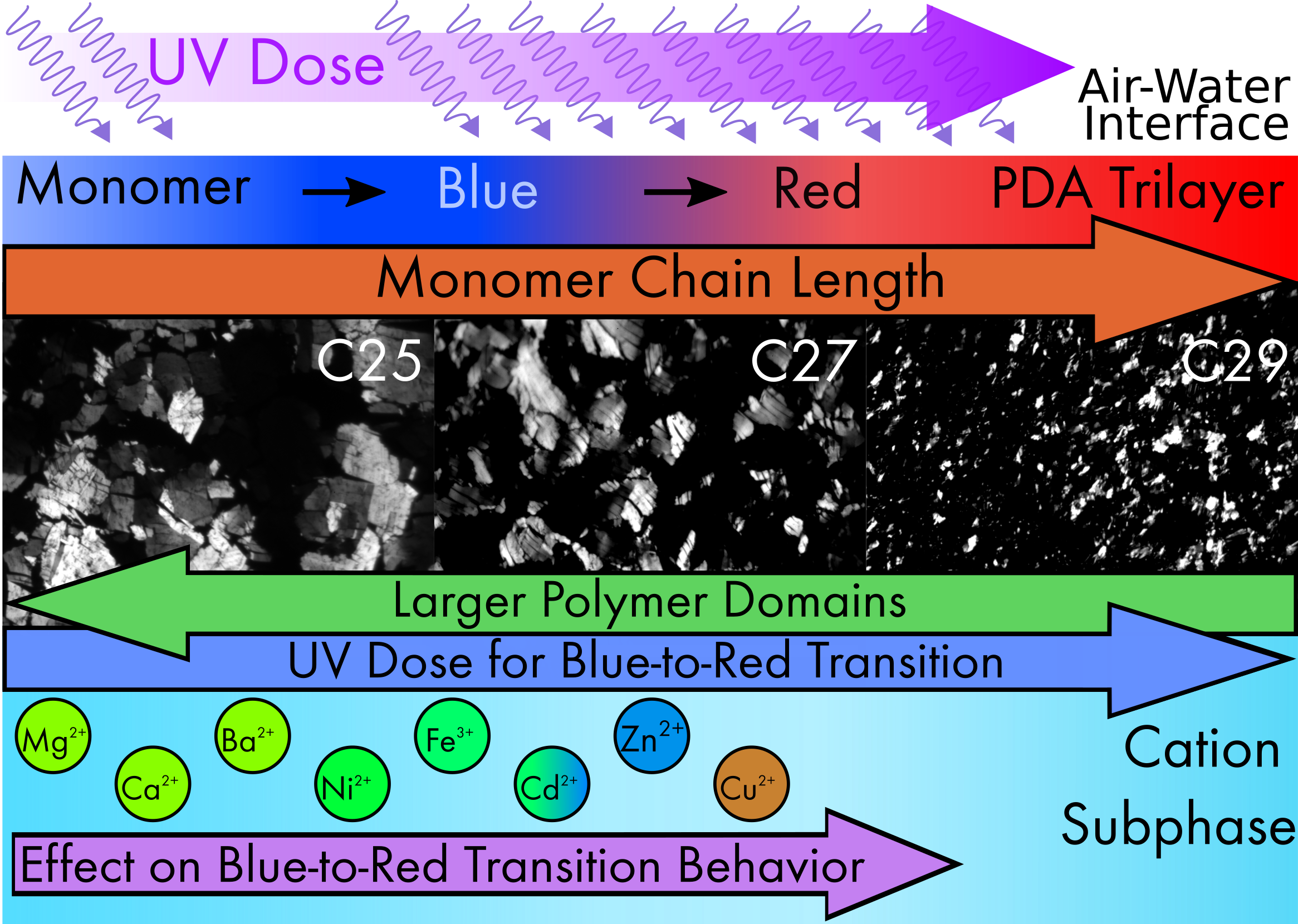
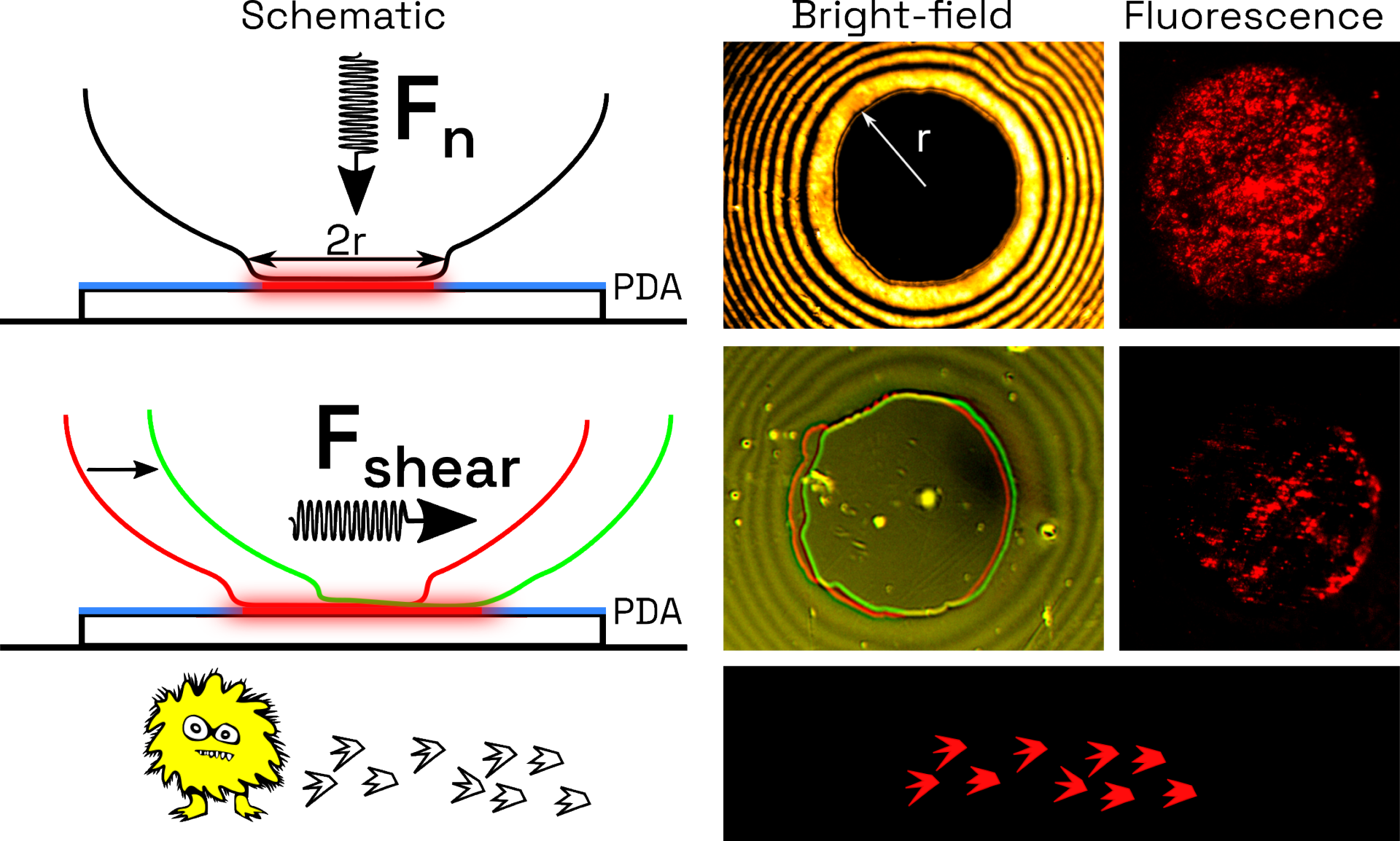
Polydiacetylenes (PDAs)
- PDAs are a class of polymers that change color from blue to red when exposed to external stimuli such as heat, light and mechanical stress
- We are developing Langmuir Films of PDAs that can be used as sensors of mechanical stress. These mechano-sensitive PDAs will be applied to quantifying cell motility and cell migratory behavior. Proof of concept demonstration was done with slime molds
- Quantifying these forces involved will provide fundamental insight into mechanisms how cells move and will enable for drug development to target cell migratory functions. This is of particular interest in pharmaceuticals that target wound healing and cancer metastasis, as cell migration is necessary for both to occur.
Integral Membrane Proteins and Nanolipoprotein Particles
An integral membrane protein (IMP) contains one or more hydrophobic domains that preferentially anchor the protein into one or both sides of the phospholipid bilayer. Varied in structure and composition, IMPs are responsible for a multitude of indispensable cell processes including, adhesion, signal transduction, and carrier mediated transport. Information regarding the structural and biophysical properties of IMPs are crucial to fields of pathology and drug development. Yet, our understanding of the functional and conformational properties of many IMPs remain obscure due to pervasive difficulties in the staging and crystallization of this unique class of biomolecules.
The folding of an IMP depends strongly upon on the complex interplay of ionic bonding, hydrogen bonding, hydrophobic, and van der Waals interactions facilitated with its host environment, making it difficult to ascertain functional conformations in non-native settings. Ongoing projects are dedicated to achieving two main goals: (i) Simulation of the optimum conditions of native membranes with polymer-supported reconstituted lipid bilayers, and (ii) delivery of IMPs into supported lipid bilayers using nanolipoprotein particles (NLPs). Using highly modular and well-defined biomimetic systems, we aim to overcome many of the obstacles associated with conventional sample preparation techniques required for high-resolution structural characterization of IMPs. Given their significant roles in cellular development, proliferation, angiogenesis, and cancer, our group focuses on two protein families: G protein-coupled receptors (GCPRs) and ErbB receptor tyrosine kinases.
This work is in collaboration with the Coleman Research Group in the University of California, Davis Department of Radiation Oncology.
Lipid Phase Behavior
Our research in cell membranes considers mechanisms such as: How do lipids partition into ordered and disordered micro-domains when self-assembled into layers? In cell membranes, how does lipid composition (type and ratio) affect interactions with embedded proteins or extracellular agents? We address these questions through computer simulations and experiments with model lipid membranes where the composition and packing of the lipids can be precisely controlled while simultaneously determining their structure, orientation, and dynamic motions.
We combine a variety of techniques to decode these phenomena. For example, a Langmuir-Blodget trough compressing a lipid monolayer at the air-water interface allows the lipids’ thermodynamic environment to be controlled explicitly while fluorescence microscopy simultaneously images the size and shape of domains in the condensed phase.
In addition, we have pioneered what is the first successful application of X-rays in characterizing the structure of supported lipid bilayers at the solid-liquid interface. Through novel environmental and experimental controls, we have been able to adapt two common scattering techniques-X-ray grazing incidence diffraction (GID) and specular reflectivity (XR)-to probe the thickness of soft biological materials with roughly twice the resolution previously accessible with other techniques.
Nanoassembly and smart films
Our multidisciplinary research has inspired several innovations in nanotechnology, such as a method for the smart assembly of nanoparticles into novel hierarchical structures, including photonic crystals, remote biosensors, and thin films with tailored optical and electrical properties. By combining polymer self-assembly and semi-conductor photolithography techniques, we are developing novel platform technologies for high throughput screening of biomolecules and other MEMS applications.
Neutron/X-Ray Scattering in Thin Layers at Interfaces
To study features smaller than the wavelength of light, we use two experimental techniques that utilize the scattering of x-rays and neutrons: X-ray/neutron reflectivity and X-ray grazing incidence diffraction (GID). Parameters such as layer thickness, density, and interfacial roughness can be determined by characterizing the surface’s reflectivity-the intensity ratio of neutrons or X-rays scattered from the surface relative to the intensity of the incident beam. X-ray GID allows the characterization of thin crystalline layers at an interface and can provide in-plane (lateral) structural information.
Other active areas of research that someone needs to write a nice blurb for the website:
- Black Lipid Membranes (BLMs) / painted lipid membranes
- Silver nanoparticles in the environment
- Gel Permeation Chromatography
